Gelöste Aufgaben/JUMP/Battery: Unterschied zwischen den Versionen
Keine Bearbeitungszusammenfassung |
|||
| Zeile 3: | Zeile 3: | ||
==Scope== | ==Scope== | ||
[[Datei:JUMP-battery-block-diagram.png|mini|Our Block-Diagram]] | |||
The battery is our power-supply. Depending on the discharging current ''I<sub>B</sub>'' consumed by our drive-train, the battery will display a voltage ''U<sub>B</sub>''. | |||
We are looking for a mathematical model that accounts for discharging operations only and we are not interested in high-frequency load-idle-cycles. Long-term changes of battery-parameters are out of scope. | |||
We copy the typical specifications for a LiPo battery used for RC Model Cars to be: | |||
* total capacity: ''Q<sub>B0</sub>'' = 5000 mAh, | |||
* voltage: ''U<sub>B0</sub>'' = 11.1 V, | |||
* C-rating: 25. | |||
The C-rating refers to the rate of energy the battery can safely discharge continuously, represented in terms of current as a multiple of its overall capacity. The maximum continuous current for this battery is | |||
<math>\begin{array}{ll} | |||
I_{B,max} & = 5000 \; mAh \cdot 25 \cdot 1/h\\ | |||
& = 125 A | |||
\end{array}</math> | |||
Also we need to model the battery heat to avoid excessive temperatures ''T<sub>B</sub>'' of the battery. | |||
==Structure== | ==Structure== | ||
Our model accounts for two aspects: | |||
# power delivery and | |||
# temperature. | |||
Both aspects are related in the sense that discharging operations with high currents lead to high battery-temperatures which will reduce the effective battery capacity. | |||
The equivalent block-diagram of our model is this: | |||
The battery-current ''I<sub>B</sub>'' entails a battery voltage ''U<sub>B</sub>'' and Temperature ''T<sub>B</sub>''. | |||
'''Power Delivery'''[[Datei:JUMP-Battery.png|mini|Battery Equivalent Circuit Diagram|alternativtext=|206x206px]]We employ a standard battery model which accounts for | |||
{| class="wikitable" | |||
| | |||
* ''E'': the equilibrium potential | |||
* ''R<sub>1</sub>, R<sub>2</sub>'': two internal resistances and | |||
* ''C'': an effective capacitance. | |||
| | |||
|} | |||
In a steady-state-condition, the battery voltage ''U<sub>B</sub>'' is thus a function of the battery current ''I<sub>B</sub>''. | |||
'''Temperature''' | |||
Our simple lumped-body model accounts for heat generation inside the battery and heat dissipation over its surface. | |||
In steady-state condition, they are equal, if heat generation <math>\dot{Q}_H</math> exceeds heat dissipation <math>\dot{Q}_D</math>, the battery heat increases - und thus its temperature. | |||
==Model== | ==Model== | ||
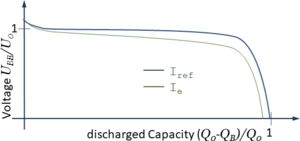
[[Datei:JUMP-battery-characteristic.png|mini|Characteristic of Electrochamical Potential.]] | [[Datei:JUMP-battery-characteristic.png|mini|Characteristic of Electrochamical Potential.]] | ||
Using Kirchhoff’s laws and from the model-diagram of the electric-circuit of the battery, we find the equilibrium conditions | |||
<math>\begin{array}{ll} | |||
U_B & = U_1 + U_2 + E(S_D) \text{ where } E(S_D) := U_{E} \text{ and }\\ | |||
I_B & = I_{C}+I_{R2} | |||
\end{array}</math>. | |||
The potential ''E'' is a function of the effective battery capacity or the commonly used dimensionless “state of dischange” | |||
<math>S_D = \frac{\displaystyle Q_{B0}-Q_B(t)}{\displaystyle Q_{B0}}</math>. | |||
''E(S<sub>D</sub>)'' depends in a complex way on the electrochemical processes within the battery. We account for this functional relationship using a characteristic curve which we copy from literature: | |||
In operation, the reference battery capacity ''Q<sub>B0</sub>'' is being consumed by the current ''I<sub>B</sub>'', thus | |||
<math>\dot{Q}_B = -\alpha(I_B) \cdot \beta(T_B) \cdot I_B</math>, | |||
where ''α'' is a correction-factor accounting for the current ''I<sub>B</sub>'' and ''β'' accounting for the battery-temperature ''T<sub>B</sub>''. The effect of ''α'' can be seen in the diagram above where ''I<sub>e</sub>'' > ''I<sub>ref</sub>''. | |||
For a representation of this function, we choose an appropriate polynomial with | |||
<math>\begin{array}{lcl} | |||
e(S_D) &:= &\frac{\displaystyle E(Q_B)}{\displaystyle U_0}\\ | |||
&=&\displaystyle \sum_0^4 C_i \cdot S_D^i | |||
\end{array}</math> | |||
Its unknown coefficients ''C<sub>i</sub>'' will be identified from the characteristic above. The components of the electric circuit provide these equations | |||
<math>\begin{array}{ll} | |||
U_1 &= R_1\cdot I_B,\\ | |||
U_2 &= R_2\cdot I_{R2},\\ | |||
I_C &= C\cdot \frac{\displaystyle d U_2}{\displaystyle dt}. | |||
\end{array}</math> | |||
with ''α'', ''β'' being current- and temperature-related correction factors respectively. | |||
With the nominal battery capacity ''Q<sub>B0</sub>'' and the actual capacity ''Q<sub>B</sub>'', we have the commonly employed state of discharge ''S<sub>D</sub>'' to be | |||
<math>\begin{array}{ll} | |||
S_D &= \frac{\displaystyle 1}{\displaystyle Q_{B0}} \cdot \displaystyle \int_0^t | |||
\alpha(I_B)\cdot \beta(T)\cdot I_B dt\\ | |||
&= 1 - \frac{\displaystyle Q_B(t)}{\displaystyle Q_{B0}}. | |||
\end{array}</math> | |||
{{MyCodeBlock | {{MyCodeBlock | ||
|title=Power Delivery | |title=Power Delivery | ||
| Zeile 27: | Zeile 111: | ||
}} | }} | ||
The thermodynamic balance-equation of heat yields | |||
<math>\dot{Q}_T = \dot{Q}_H - \dot{Q}_D</math>. | |||
{| class="wikitable" | |||
|''Note that Q is used here as the heat of the battery – not as the battery-capacity as above!'' | |||
|} | |||
The battery heat is a function of | |||
* temperature ''T<sub>B</sub>,'' | |||
* averaged specific heat capacity ''c<sub>p,B</sub>'' and | |||
* battery mass ''m<sub>B</sub>''. | |||
so that | |||
<math>\dot{Q}_T = m_B\cdot c_B\cdot \frac{\displaystyle d T}{\displaystyle dt}</math>. | |||
Heat generation inside the battery be | |||
<math>\dot{Q}_H = R_1\cdot I_B^2+R_2\cdot I_{R2}^2</math>. | |||
and heat dissipation | |||
<math>\dot{Q}_D = h_B\cdot (T_B-T_0)</math> | |||
with ambient air temperature ''T<sub>0</sub>'' and the coefficient of heat transfer ''h<sub>B</sub>''.[[Datei:JUMP-battery-02.png|mini|Battery Heat Rates.|alternativtext=|123x123px]] | |||
{{MyCodeBlock | {{MyCodeBlock | ||
| Zeile 36: | Zeile 147: | ||
</syntaxhighlight> | </syntaxhighlight> | ||
}} | }} | ||
== Summary == | |||
Thus, the battery-coordinates are | |||
<math>\underline{q}_B = \left( | |||
\begin{array}{c} | |||
Q_B(t)\\ | |||
U_{2}(t)\\ | |||
T_B(t) | |||
\end{array} | |||
\right)</math> | |||
with the associated equations of motion | |||
<math>\begin{array}{ll} | |||
\dot{Q}_B &= - \alpha(I_B)\cdot \beta(T)\cdot I_B \text{ and } S_D &= \frac{\displaystyle Q_B(t) - Q_{B0}}{\displaystyle Q_{B0}},\\\dot{U}_2 &= \frac{\displaystyle I_C}{\displaystyle C},\\ | |||
\dot{T}_B &= \frac{\displaystyle 1}{\displaystyle m_B \; c_B} \cdot \left( \dot{Q}_H - \dot{Q}_D\right)\\ | |||
\end{array}</math> | |||
and additional algebraic equation | |||
<math>\begin{array}{ll} | |||
\dot{Q}_H &= R_1\cdot I_B^2 + R_{R2} \cdot I_2^2 \text{ where } I_{R2} = \frac{\displaystyle U_2}{\displaystyle R_2}\\ | |||
U_B &= R_1 \cdot I_B + U_{2} + E(S_D),\\ | |||
\dot{Q}_D &= H_B \cdot (T_B(t) - T_0). | |||
\end{array}</math> | |||
== Variables == | |||
{| class="wikitable" | |||
!name | |||
!symbol | |||
!unit | |||
|- | |||
|battery remaining capacity | |||
|''Q<sub>B</sub>'' | |||
|A h | |||
|- | |||
|capacitance voltage | |||
|''U<sub>B2</sub>'' | |||
|V | |||
|- | |||
|battery temperature | |||
|''T<sub>B</sub>'' | |||
|°C | |||
|} | |||
== Parameters == | |||
The coefficients ''C<sub>i</sub>'' for ''e(S<sub>D</sub>)'' have been computed from conditions for a 5th-order polynomial by defining function-values and gradients at reference points: | |||
<math>C_0=\frac{11}{10},C_1=-1,C_2=\frac{1177}{240},C_3=-\frac{28489}{2160},C_4=\frac{7891}{432},C_5=-\frac{4355}{432}</math>[[Datei:JUMP-battery-characteristic-implemented.png|mini|Implemented Characteristic]]The plot right shows the resultant characteristic of ''e(S<sub>D</sub>)'': | |||
For the remaining parameters, no measurements were available. We adopted those to obtain a performance similar to what has been described in [1], the resistors were chosen as to “deliver” reasonable amounts of heat when the battery is discharged with rating of C=25. | |||
* ''R<sub>1</sub>'' = 500 mΩ | |||
* ''R<sub>2</sub>'' = 250 mΩ | |||
* C = 500 F | |||
* ''Q<sub>B0</sub>'' = 5000 Ah | |||
* ''T<sub>0</sub>'' = 20°C | |||
For ''α'' and ''β'' we know that their gradients | |||
<math>\frac{\displaystyle d \; \alpha}{ \displaystyle d \; I_B} > 0</math> | |||
and | |||
<math>\frac{\displaystyle d\; \beta}{\displaystyle \; dT} < 0</math>. | |||
The reference curve from above shall be assumed to be valid for ''I<sub>B</sub>'' = 20 A and we assume | |||
<math>\begin{array}{lll} | |||
\alpha(I_B) &= 1 + \frac{\displaystyle I_B}{\displaystyle I_ \alpha} &\text{ with } I_\alpha = 100 A\\ | |||
\beta(T) &= 1 - \frac{\displaystyle T_B}{\displaystyle T_ \beta} &\text{ with } T_ \beta = 100^\circ C | |||
\end{array}</math> | |||
Then, for the thermal model we have | |||
* ''m<sub>B</sub>'' = 340 g, | |||
* ''c<sub>p,B</sub>'' = 2 J/(kg K). | |||
And finally ''h<sub>B</sub>'' will be identified from a (virtual) experiment: let ''I<sub>B</sub>'' = 0, ''I<sub>2</sub>''=0 (no heat generation) and the initial battery-temperature to be ''ΔT(0)'' above ''T<sub>0</sub>''. Then assume that ''ΔT(''3min'')'' is reduced to 50% of ''ΔT(0)''. From above, we find the differential equation to be | |||
<math>\Delta T(t)= \Delta T_0 \cdot \text{e}^{-\frac{\displaystyle h_B \cdot t}{\displaystyle m_B \cdot c_{PB}}}</math>, | |||
thus for this “experiment” | |||
<math>h_B=(\text{log}(2) \cdot m_B\cdot c_{PB})/ 3 \text{ min}</math>. | |||
With the values given above, we find | |||
''h<sub>B</sub>=2.6E-3 J/(K''s) | |||
Let the tolerable, maximum battery temperature be | |||
<math>T_{B,max} = 60^\circ C</math>. | |||
[[Gelöste Aufgaben/JUMP/Car-Body|next workpackage: car-body →]] | [[Gelöste Aufgaben/JUMP/Car-Body|next workpackage: car-body →]] | ||
| Zeile 41: | Zeile 247: | ||
'''References''' | '''References''' | ||
# Lijun Gao, Shengyi Liu; Roger A. Dougal: Dynamic Lithium-Ion Battery Model for System Simulation, IEEE TRANSACTIONS ON COMPONENTS AND PACKAGING TECHNOLOGIES, VOL. 25, NO. 3, SEPTEMBER 2002 pp. 495 | |||
# P. Villano, M. Carewska, S. Passerini: Specific heat capacity of lithium polymer battery components; Elsevier, Thermochimica Acta 402 (2003) 219–224 | |||
Version vom 10. März 2021, 09:58 Uhr
Scope
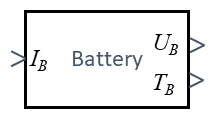
The battery is our power-supply. Depending on the discharging current IB consumed by our drive-train, the battery will display a voltage UB.
We are looking for a mathematical model that accounts for discharging operations only and we are not interested in high-frequency load-idle-cycles. Long-term changes of battery-parameters are out of scope.
We copy the typical specifications for a LiPo battery used for RC Model Cars to be:
- total capacity: QB0 = 5000 mAh,
- voltage: UB0 = 11.1 V,
- C-rating: 25.
The C-rating refers to the rate of energy the battery can safely discharge continuously, represented in terms of current as a multiple of its overall capacity. The maximum continuous current for this battery is
Also we need to model the battery heat to avoid excessive temperatures TB of the battery.
Structure
Our model accounts for two aspects:
- power delivery and
- temperature.
Both aspects are related in the sense that discharging operations with high currents lead to high battery-temperatures which will reduce the effective battery capacity.
The equivalent block-diagram of our model is this:
The battery-current IB entails a battery voltage UB and Temperature TB.
Power Delivery
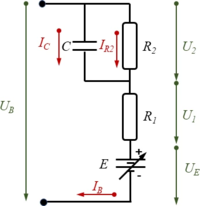
We employ a standard battery model which accounts for
|
In a steady-state-condition, the battery voltage UB is thus a function of the battery current IB.
Temperature
Our simple lumped-body model accounts for heat generation inside the battery and heat dissipation over its surface.
In steady-state condition, they are equal, if heat generation exceeds heat dissipation , the battery heat increases - und thus its temperature.
Model
Using Kirchhoff’s laws and from the model-diagram of the electric-circuit of the battery, we find the equilibrium conditions
.
The potential E is a function of the effective battery capacity or the commonly used dimensionless “state of dischange”
.
E(SD) depends in a complex way on the electrochemical processes within the battery. We account for this functional relationship using a characteristic curve which we copy from literature:
In operation, the reference battery capacity QB0 is being consumed by the current IB, thus
,
where α is a correction-factor accounting for the current IB and β accounting for the battery-temperature TB. The effect of α can be seen in the diagram above where Ie > Iref.
For a representation of this function, we choose an appropriate polynomial with
Its unknown coefficients Ci will be identified from the characteristic above. The components of the electric circuit provide these equations
with α, β being current- and temperature-related correction factors respectively.
With the nominal battery capacity QB0 and the actual capacity QB, we have the commonly employed state of discharge SD to be
Power Delivery
Text
1+1=2
The thermodynamic balance-equation of heat yields
.
| Note that Q is used here as the heat of the battery – not as the battery-capacity as above! |
The battery heat is a function of
- temperature TB,
- averaged specific heat capacity cp,B and
- battery mass mB.
so that
.
Heat generation inside the battery be
.
and heat dissipation
with ambient air temperature T0 and the coefficient of heat transfer hB.

Temperature
Text
1+1=2
Summary
Thus, the battery-coordinates are
with the associated equations of motion
and additional algebraic equation
Variables
| name | symbol | unit |
|---|---|---|
| battery remaining capacity | QB | A h |
| capacitance voltage | UB2 | V |
| battery temperature | TB | °C |
Parameters
The coefficients Ci for e(SD) have been computed from conditions for a 5th-order polynomial by defining function-values and gradients at reference points:
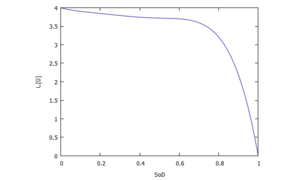
The plot right shows the resultant characteristic of e(SD):
For the remaining parameters, no measurements were available. We adopted those to obtain a performance similar to what has been described in [1], the resistors were chosen as to “deliver” reasonable amounts of heat when the battery is discharged with rating of C=25.
- R1 = 500 mΩ
- R2 = 250 mΩ
- C = 500 F
- QB0 = 5000 Ah
- T0 = 20°C
For α and β we know that their gradients
and
.
The reference curve from above shall be assumed to be valid for IB = 20 A and we assume
Then, for the thermal model we have
- mB = 340 g,
- cp,B = 2 J/(kg K).
And finally hB will be identified from a (virtual) experiment: let IB = 0, I2=0 (no heat generation) and the initial battery-temperature to be ΔT(0) above T0. Then assume that ΔT(3min) is reduced to 50% of ΔT(0). From above, we find the differential equation to be
,
thus for this “experiment”
.
With the values given above, we find
hB=2.6E-3 J/(Ks)
Let the tolerable, maximum battery temperature be
.
References
- Lijun Gao, Shengyi Liu; Roger A. Dougal: Dynamic Lithium-Ion Battery Model for System Simulation, IEEE TRANSACTIONS ON COMPONENTS AND PACKAGING TECHNOLOGIES, VOL. 25, NO. 3, SEPTEMBER 2002 pp. 495
- P. Villano, M. Carewska, S. Passerini: Specific heat capacity of lithium polymer battery components; Elsevier, Thermochimica Acta 402 (2003) 219–224